The Medical Device Coordination Group (MDCG) has released revised guidance documents, MDCG 2024-2 Rev.1 and 2021-12 Rev.1, focusing on the European Medical Device Nomenclature (EMDN). This update, released in January 2025, provides critical clarifications and insights for medical device manufacturers, aiming to ensure accurate and consistent use of the EMDN system.
What is the EMDN, and how does it affect me?
The EMDN is a standardised system for classifying and identifying medical devices within the European Union. Introduced under Article 26 of the Medical Device Regulation (MDR) (EU) 2017/745 and Article 23 of the In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746, the EMDN plays a crucial role in the functioning of the European Database on Medical Devices (EUDAMED).
Manufacturers utilise the EMDN to register their devices in EUDAMED, linking each device with a Unique Device Identifier - Device Identifier (UDI-DI). Beyond registration, the EMDN is vital for device documentation, notified body assessments, post-market surveillance, vigilance activities, and data analysis. It serves as a key reference for all stakeholders, including patients, providing clear device descriptions and promoting transparency in the medical device market. The EMDN is reviewed and updated annually based on user feedback and practical use.
Key changes at a glance
| Update | Description |
|---|---|
| New and revised FAQs | The guidance document includes a restructured FAQ section with new questions and clarifications on various aspects of the EMDN. |
| Code granularity | Manufacturers are reminded to use the most specific and detailed (terminal) EMDN code available for each device. |
| Multiple codes | Clearer instructions are provided for using multiple EMDN codes for devices with multiple intended purposes, especially complex systems. |
| Code gaps | A process for proposing new EMDN codes when existing codes are not suitable is outlined, ensuring the nomenclature stays current. |
| Annual review and updates | The importance of staying informed about annual EMDN updates and making necessary changes to device registrations and documentation is emphasised. |
| Obsolete and split codes | Clear procedures for managing obsolete and split codes in EUDAMED are provided, including archiving and public accessibility. |
The updated guidance document features a restructured FAQ section with new questions addressing specific aspects of EMDN implementation and management. Some of the key areas covered include:
- EMDN Code Assignment: The document emphasises assigning the most granular and terminal code available to each device in EUDAMED. It clarifies the use of multiple codes for devices with multiple intended purposes, particularly complex systems with diverse functions.
- Handling Code Gaps: If an appropriate EMDN term is not available, manufacturers are advised to use the code extension ‘99’ (‘Other’) and submit a proposal for a new code. This ensures the EMDN stays current with technological advancements and evolving device types.
- Annual EMDN Review: The update highlights the annual EMDN update cycle and the importance of timely updates to EUDAMED entries and related documentation. Manufacturers are encouraged to review the updated EMDN annually to identify any changes impacting their devices and notify their notified bodies accordingly.
- Code Updates and EUDAMED: The guidance provides clear instructions on handling obsolete or split codes in EUDAMED. Obsolete codes remain visible for a period, allowing for a smooth transition, while split codes are archived and made publicly accessible.
Annual revision process
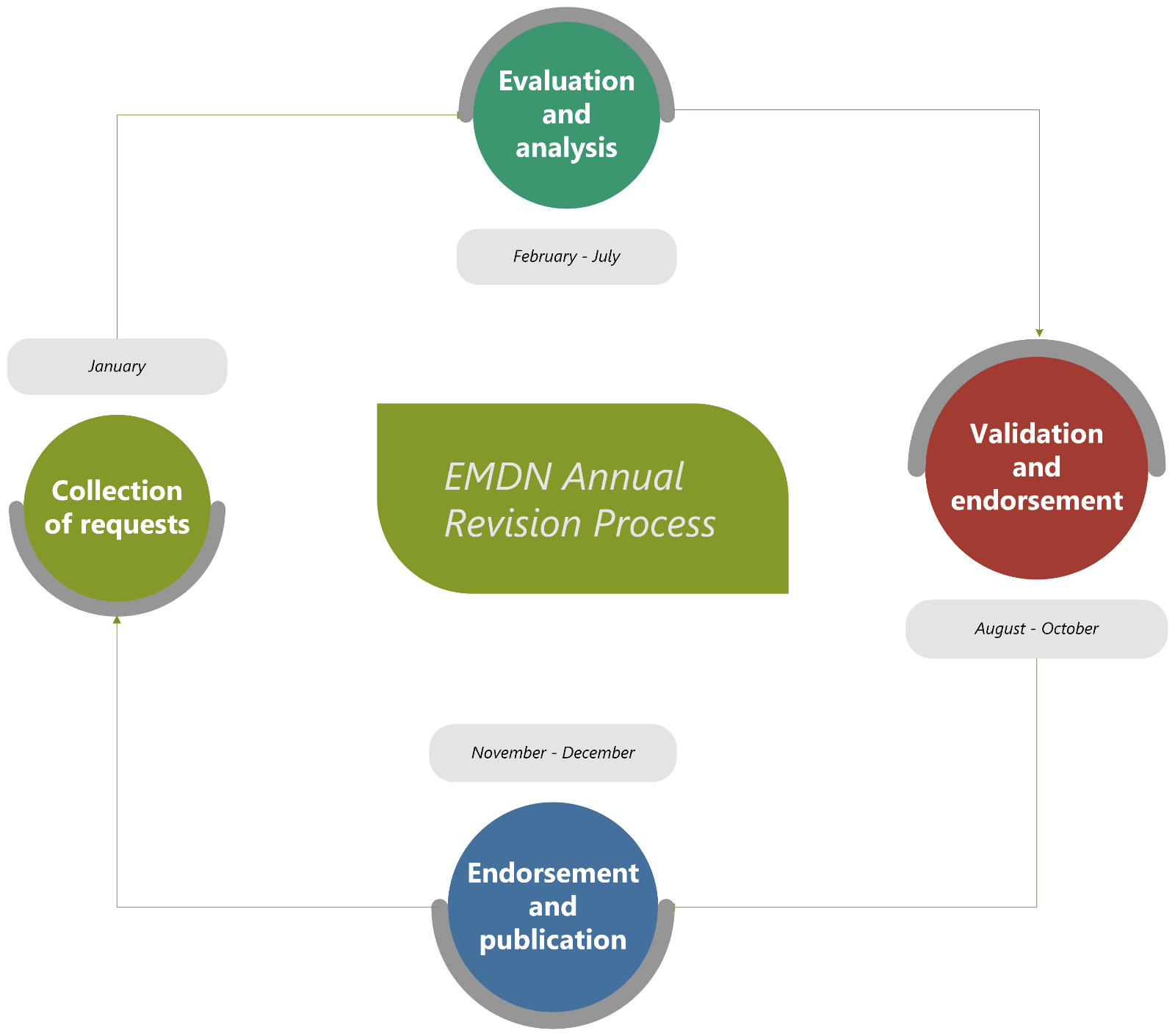
MDCG 2024-2 Rev.1 contains a detailed explanation of the annual EMDN revision process. The most important part of the process for manufacturers is the collection of requests step. EMDN users, including competent authorities, notified bodies, manufacturers, and other stakeholders, can submit requests for changes to the EMDN through a dedicated platform. The deadline for submitting requests for processing in that same year is 31st January.
These requests are then reviewed by the EMDN Technical Team (EMDN-TT) and the MDCG Nomenclature Working Group (NOM WG) over the course of the year before finally being published to EUDAMED by the end of the year.
In addition to the annual update cycle, a pilot procedure for ad-hoc updates requiring expedited review has been introduced. This ad-hoc process is limited to new code requests and is only available to competent authorities and notified bodies.
What does this mean for me?
This update underscores the importance of staying informed about EMDN changes and ensuring accurate EMDN code assignment for all medical devices. Manufacturers must proactively engage with the annual EMDN review process, participate in code update proposals, and maintain accurate device information in EUDAMED.
By adhering to these guidelines manufacturers can:
- Enhance Regulatory Compliance: Accurate EMDN coding is crucial for meeting regulatory requirements and avoiding potential issues with notified bodies and market access.
- Improve Data Quality: Consistent and precise EMDN coding contributes to the quality and reliability of data in EUDAMED, supporting effective post-market surveillance and regulatory decision-making.
- Increase Transparency: Clear and standardised device identification through the EMDN promotes transparency for patients, healthcare professionals, and other stakeholders.
Conclusion
The MDCG have provided valuable guidance for medical device manufacturers navigating the EMDN system. By understanding and implementing these guidelines, manufacturers can ensure accurate device classification, maintain regulatory compliance, and contribute to a transparent and well-functioning medical device market.
Navigating the complexities of EMDN and EUDAMED can be challenging. Partnering with Mantra Systems allows you to focus on your core business while we handle the intricacies of EMDN compliance. Book a free, no-obligation chat with one of our regulatory experts to see how we can help.